
#Keynote 042 trial#
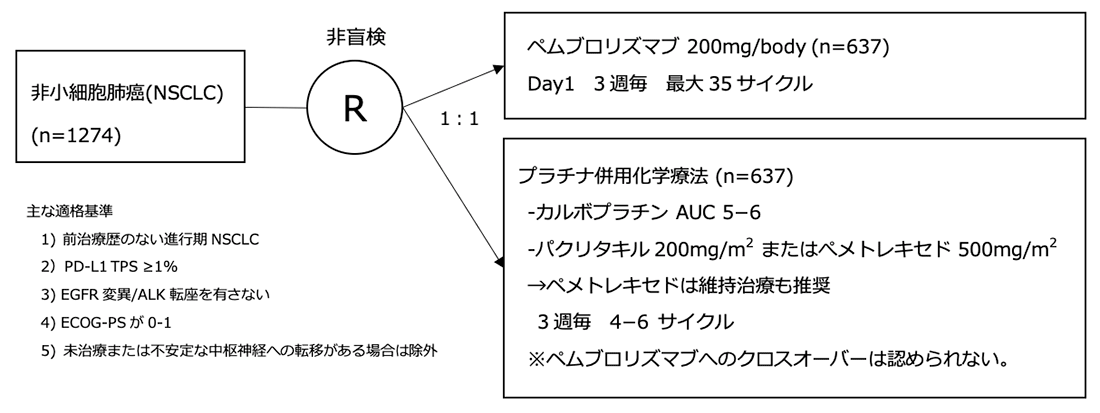
The trial headline was that pembrolizumab monotherapy might be an additional effective option for this patient population ( 14). KEYNOTE-042 compared pembrolizumab to histology dependent platinum-based doublets as initial treatment in locally advanced or metastatic patients with PD-L1 ≥1% on TCs and lacking EGFR activating mutations/ ALK fusions.
#Keynote 042 plus#
Until recently, the recommended first-line therapy by most guideline panels and regulatory agencies for metastatic NSCLC patients lacking an approved targeted therapy option has been pembrolizumab monotherapy or chemo-immunotherapy (ABCP or pembrolizumab plus histology dependent chemotherapy) if PD-L1 was ≥50% on TCs and chemo-immunotherapy if PD-L1 was <50% on TCs. CheckMate-026, which compared nivolumab to histology dependent platinum-based doublets in patients without EGFR activating mutations/ ALK fusions, failed to demonstrate improved OS for nivolumab in the overall study population with PD-L1 ≥5% on TCs or in the subgroup with PD-L1 ≥50% on TCs ( 13). Similarly, within KEYNOTE-407, pembrolizumab plus platinum/taxane demonstrated improved ORR (57.9% vs. 48%), PFS (HR 0.62, P<0.001) and OS (HR 0.78, P=0.02) for non-squamous NSCLC patients regardless of PD-L1 staining when compared to carboplatin plus paclitaxel plus bevacizumab ( 12). In IMpower150, the regimen of ABCP also demonstrated improved ORR (63.5% vs. 18.9%, P<0.001), PFS and OS (HR 0.49, P<0.001) than platinum/pemetrexed across non-squamous NSCLC patients with any level PD-L1 staining (including PD-L1 negative patients) ( 10).

In KEYNOTE-189, pembrolizumab plus platinum/pemetrexed had better ORR (47.6% vs. For patients lacking EGFR activating mutations/ ALK fusions these chemo-immunotherapy combinations improved efficacy outcomes compared to platinum-based chemotherapy ( 10- 12). Following this, both atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABCP) and pembrolizumab plus histology dependent chemotherapy were compared to platinum-based chemotherapy in randomized phase III trials. Subsequently, KEYNOTE-024 demonstrated that for advanced/metastatic NSCLC patients with PD-L1 ≥50% on TCs by the 22-C3 immunohistochemistry assay and lacking EGFR activating mutations/ ALK fusions that pembrolizumab had a significantly greater objective response rate (ORR), progression-free survival (PFS) and OS when compared to platinum-based doublets as first-line therapy ( 8, 9).īecause of KEYNOTE-024, multiple regulatory agencies and guideline panels approved pembrolizumab monotherapy for patients with newly diagnosed advanced/metastatic NSCLC, PD-L1 ≥50% on TCs and lacking EGFR activating mutations/ ALK fusions. In each of these second-line trials patients with epidermal growth factor receptor (EGFR) activating mutations did not benefit from PD-1 axis inhibitors when compared to docetaxel, with data on any included ALK rearranged patients not detailed ( 4- 7). Within KEYNOTE-010, patients with PD-L1 ≥50% on tumor cells (TCs) administered pembrolizumab had a greater magnitude of benefit compared to docetaxel than patients with PD-L1 of 1–49% ( 6). In each of these trials, there was an OS benefit when comparing the respective PD-1 or PD-L1 inhibitor to docetaxel ( 4- 7). Subsequently, randomized phase III trials comparing atezolizumab, nivolumab or pembrolizumab to docetaxel as second-line therapy were conducted in NSCLC.

Prior to the advent of inhibitory antibodies targeting programmed death 1 (PD-1) or programmed death ligand 1 (PD-L1), the median overall survival (OS) for advanced/metastatic non-small cell lung cancer (NSCLC) patients receiving first-line platinum doublet chemotherapy was approximately 8–12 months and 5-year survival rates were estimated at 2% ( 1- 3). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Email: This is an invited article commissioned by the Section Editor Kaiping Zhang, PhD (AME College, AME Group, China).Ĭomment on: Mok TSK, Wu YL, Kudaba I, et al. Thoracic Oncology Program, Division of Medical Oncology, Department of Internal Medicine, University of Colorado Cancer Center, Aurora, CO, USA. Interviews with Outstanding Guest EditorsĬorrespondence to: Jose M.Policy of Dealing with Allegations of Research Misconduct.Policy of Screening for Plagiarism Process.


 0 kommentar(er)
0 kommentar(er)
